ECDOC_100263117.pdf
MDD_DoC_Suzhou Avon Textile Co.; Ltd_OR Towel nonsterile
Foliodrape® products are to prevent the passage of pathogens between non-sterile and sterile areas.
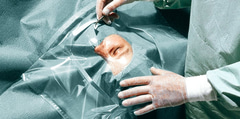
Foliodrape® Ophthalmology sets sterile are single-use products for short-term use and consists of a variety of products like patient drapes, equipment covers, fixation and collection accessories, commodity products (e.g. towels); included into sterile packages. The Foliodrape® standard sets are intended to be used in different fields of applications/disciplines. Foliodrape® products are to prevent the passage of pathogens between non-sterile and sterile areas. The Polyethylene-film or different layers of hydrophilic nonwoven material laminated with Polyethylene-film or different layers of hydrophobic nonwoven material (SMS) act together as a fluid and bacteria barrier and minimize the transmission of micro-organisms. These products are placed onto the market sterile and are in Medical Device Class I.
Estéril, embalagem individual
| Informação do produto | Código de artigo | Conteúdo | EAN | ||
| Estéril, embalagem individual | |||||
|
1 Caixa desdobrável com 10 sets 1 Caixa com 2 Caixas desdobráveis | 4052199211992 4052199212005 | |||