ECDOC_100393581.pdf
PHAG_Cons. EU DoC_MDR_Class I
Zetuvit E sterile is made of smooth cellulose fibers with high absorption capacity. Its water-repellent backing prevents strikethrough and protects clothing and bedding from wound exudate. The absorbent core of the dressing is surrounded by a cellulosic tissue layer enhancing the distribution of exudate and the whole is wrapped in a non-adherent and hydrophilic nonwoven. An easy application is ensured by a blue-coloured thread on the backside of the product. Due to its excellent padding properties, extraordinary softness and adaptability to body contours Zetuvit E sterile provides high wearing comfort for the patient.


sterile, individually sealed
| Product Information | Article Number | Content | EAN | ||
| sterile, individually sealed | |||||
| 10x10 cm |
|
1 Folding box of 25 pieces 1 Box of 9 Folding boxes | 4049500996068 4049500996075 | ||
| 10x20 cm |
|
1 Folding box of 25 pieces 1 Box of 9 Folding boxes | 4049500996082 4049500996099 | ||
| 20x20 cm |
|
1 Folding box of 15 pieces 1 Box of 7 Folding boxes | 4049500996143 4052199100104 | ||
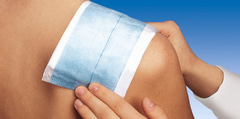
Zetuvit E sterile can be used
Zetuvit E sterile can remain in place for several days and needs to be fixed with appropriate fixation material.