ECDOC_100393581.pdf
PHAG_Cons. EU DoC_MDR_Class I
Good cushioning properties
Air-permeable / breathable

Stérile, emballage individuel
Non stérile
| Information produit | Numéro d'article | Contenu | EAN | ||||
| Stérile, emballage individuel | |||||||
| 56mm x 70mm |
|
1 Boîte pliante de 25 pièces 1 Carton de 12 Boîtes pliantes | 4049500800969 4049500901086 | ||||
| 70mm x 85mm |
|
1 Boîte pliante de 25 pièces 1 Carton de 12 Boîtes pliantes | 4049500800976 4049500901093 | ||||
| Non stérile | |||||||
| 56mmx70mm |
|
1 Boîte pliante de 50 pièces 1 Carton de 15 Boîtes pliantes | 4049500800631 4049500901123 | ||||
| 70 x 85 mm |
|
1 Boîte pliante de 50 pièces 1 Carton de 15 Boîtes pliantes | 4049500800648 4049500901130 | ||||
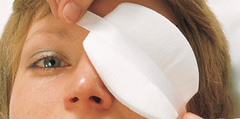
Eycopad non-sterile
The primary function of the non-sterile Eycopad is to act as mechanical barrier to protect the eye/outer eye region and to absorb body fluids. It can be used as carrier for externas.
Application of liquids and ointments on the eye.
Mechanical barrier for wound protection after intervention (padding effect).
Contraindications: Surgical wounds
Eycopad sterile
The primary function of the device is to act as mechanical barrier to protect the eye/outer eye region and to absorb body fluids, covering of dry and weeping wounds, protection and padding of the eye, only for external application. It can be used as carrier for externas.
Wound protection after intervention (padding effect)
Injury of the outer eye region: Application of externas e.g. liquids and ointments on the outer eye region, weeping and dry wounds
Contraindications: Surgical wounds