ECDOC_100263117.pdf
MDD_DoC_Suzhou Avon Textile Co.; Ltd_OR Towel nonsterile
Foliodrape® producten zijn bedoeld om de doorgang van ziekteverwekkers tussen niet-steriele en steriele zones te voorkomen.
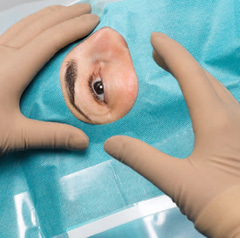
Foliodrape® Oogheelkundige sets steriel zijn producten voor eenmalig gebruik voor kortdurend gebruik en bestaan uit een verscheidenheid aan producten zoals patiëntdoeken, afdekdoeken voor apparatuur, fixatie- en opvangaccessoires, basisproducten (bijv. handdoeken); opgenomen in steriele verpakkingen. De Foliodrape® standaardsets zijn bedoeld voor gebruik in verschillende toepassingsgebieden/disciplines. Foliodrape® producten voorkomen de doorgang van ziekteverwekkers tussen niet-steriele en steriele zones. De polyethyleenfolie of verschillende lagen hydrofiel nonwoven materiaal gelamineerd met polyethyleenfolie of verschillende lagen hydrofoob nonwoven materiaal (SMS) werken samen als een vloeistof- en bacteriebarrière en minimaliseren de overdracht van micro-organismen. Deze producten worden steriel op de markt gebracht en vallen onder Medical Device Class I.
De set bevat alle items die nodig zijn voor het afdekken van de patiënt.
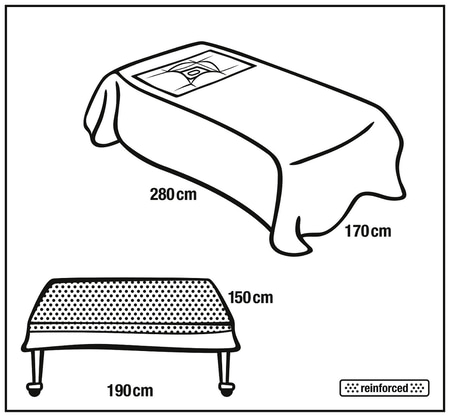
Steriel, per stuk verpakt
| Productinformatie | Artikelnummer | Inhoud | EAN | ||
| Steriel, per stuk verpakt | |||||
|
1 verpakking van 7 sets 1 omdoos van 1 verpakking | 4052199523392 4052199523385 | |||
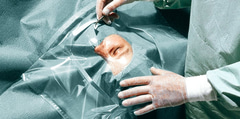
Als steriele afdekset bij oogchirurgie, bijv. bij lamellaire of geperforeerde keratoplastiek, intracapsulaire of extracapsulaire cataractextracties, transconjunctivale cryocoagulatie, cerclage van de oogbol, parsplana-phacofragmentatie, orbitale exenteratie.