ECDOC_100393581.pdf
PHAG_Cons. EU DoC_MDR_Class I
Good cushioning properties
Air-permeable / breathable

sterile, individually sealed
non sterile, in folding boxes
sterile, in folding boxes
| Informacja o produktach | Numer artykułu | Treść | EAN | ||||
| sterile, individually sealed | |||||||
| 56mm x 70mm |
|
1 op. z 25 sztuk 1 kart. zbior. z 12 op. | 4049500800969 4049500901086 | ||||
| non sterile, in folding boxes | |||||||
| 56mm x 70mm |
|
1 op. z 5 szt. 1 kart. zbior. z 105 op. | 4049500800983 4049500901109 | ||||
| 70mm x 85mm |
|
1 op. z 5 sztuk 1 kart. zbior. z 60 op. | 4049500800990 4049500901116 | ||||
| sterile, in folding boxes | |||||||
| 70mm x 85mm |
|
1 op. z 25 sztuk 1 kart. zbior. z 12 op. | 4049500800976 4049500901093 | ||||
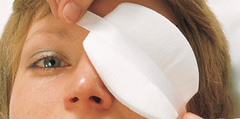
Eycopad non-sterile
The primary function of the non-sterile Eycopad is to act as mechanical barrier to protect the eye/outer eye region and to absorb body fluids. It can be used as carrier for externas.
Application of liquids and ointments on the eye.
Mechanical barrier for wound protection after intervention (padding effect).
Contraindications: Surgical wounds
Eycopad sterile
The primary function of the device is to act as mechanical barrier to protect the eye/outer eye region and to absorb body fluids, covering of dry and weeping wounds, protection and padding of the eye, only for external application. It can be used as carrier for externas.
Wound protection after intervention (padding effect)
Injury of the outer eye region: Application of externas e.g. liquids and ointments on the outer eye region, weeping and dry wounds
Contraindications: Surgical wounds